The EU MDR is a set of regulations released in 2017 by the European Union to ensure high safety and quality standards for medical devices in member countries. It replaces the previous Medical Device Directive (MDD) and aims to improve health and safety while supporting innovation. Companies have a transition period until May 2021 to comply with the new regulations. The EU MDR introduces changes, including a unique device identification system and requirements for technical documentation. It covers various classes of medical devices based on risk levels. The goal is to enhance the safety and performance of medical devices and improve overall healthcare safety.
At Tytex we are dedicated to providing optimal healthcare solutions. As part of this commitment, Tytex is actively and continuously working on implementing the findings of the EU MDR legislation framework as well as other relevant legislation. By adhering to the regulations and requirements set forth in the EU MDR and other legislation, we at Tytex aims to ensure that our medical devices meet the highest standards of safety and quality.
Implementing the EU MDR framework allows Tytex to stay up to date with the latest industry standards and best practices. By doing so, we can offer innovative
and reliable healthcare solutions to all customers while prioritizing patient safety and well-being.
By proactively embracing the EU MDR legislation, Tytex demonstrates our commitment to continuously improving our products and processes. This also includes updating technical documentation, complying with the unique device identification system, and implementing a robust Quality Management System.
Through our diligent work with implementing the EU MDR framework, we ensure that all healthcare solutions are in line with the regulatory requirements and contribute to the overall goal of enhancing the safety and performance of medical devices. By doing so, we provide healthcare professionals and patients with trusted and reliable products that meet the highest quality standards.
Below you can learn more about the symbols we employ for various material. Should you have any questions please let us know or contact our Compliance Manager at [email protected] or by phone on +45 5154 7471.
| NAME OF SYMBOL | SYMBOL | EXPLANATION OF SYMBOL |
| Manufacturer | 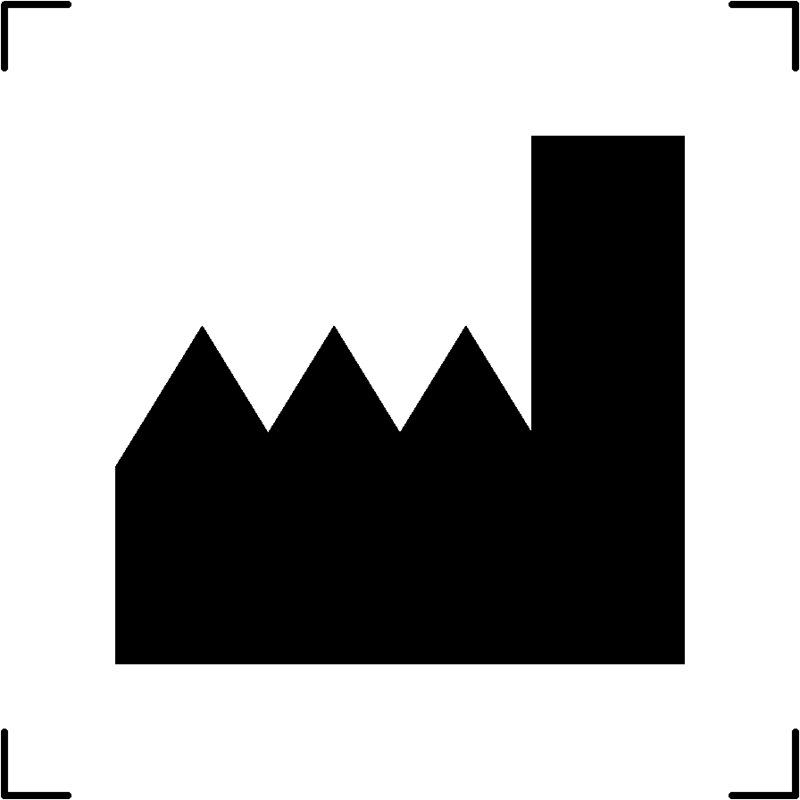 |
Who is the manufacturer |
| Country of manufacture |  |
Which country is the product manufactured in |
| Date of manufacture | 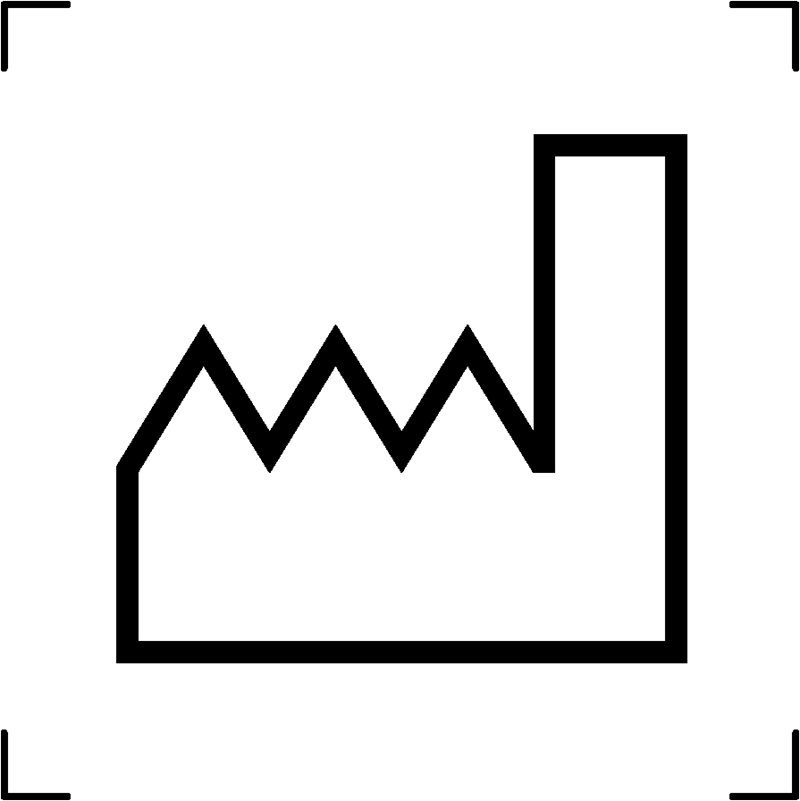 |
When is the product manufactured |
| Importer | 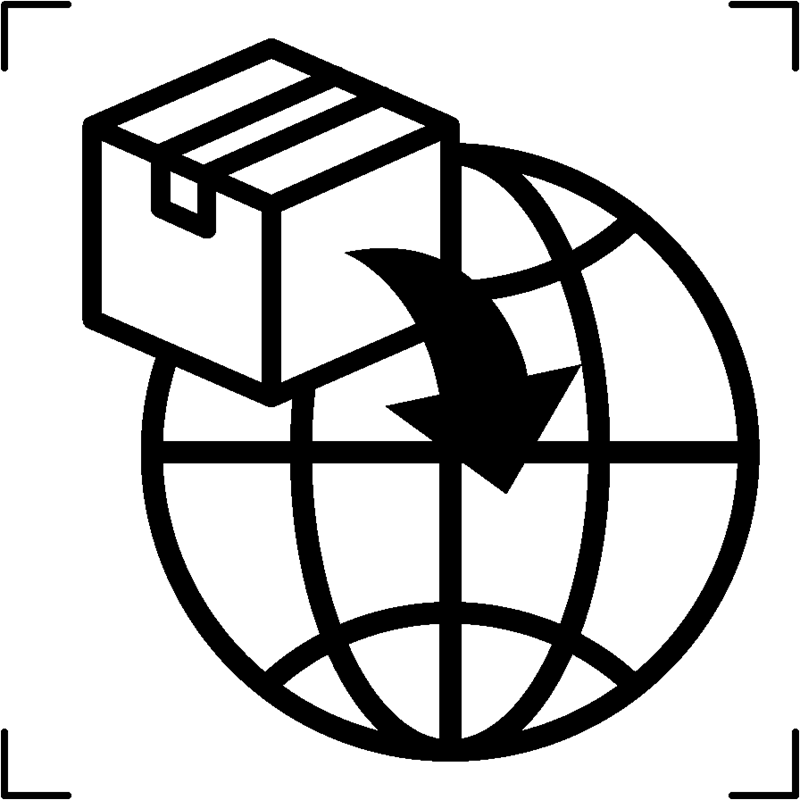 |
Who is the importer of the product |
| Distributor | 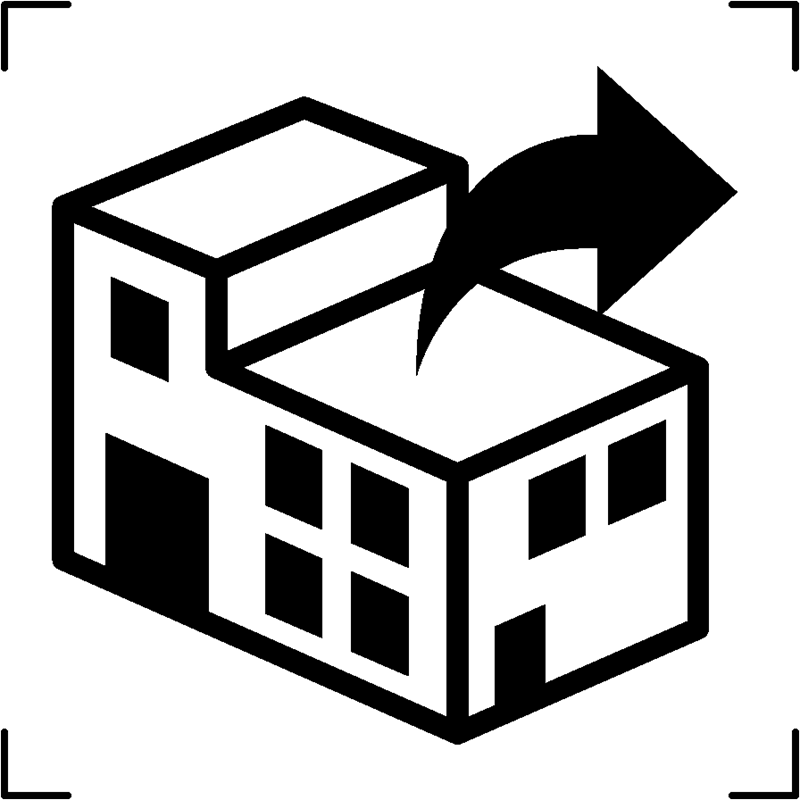 |
Who is the distributor of the product |
| Use by date | 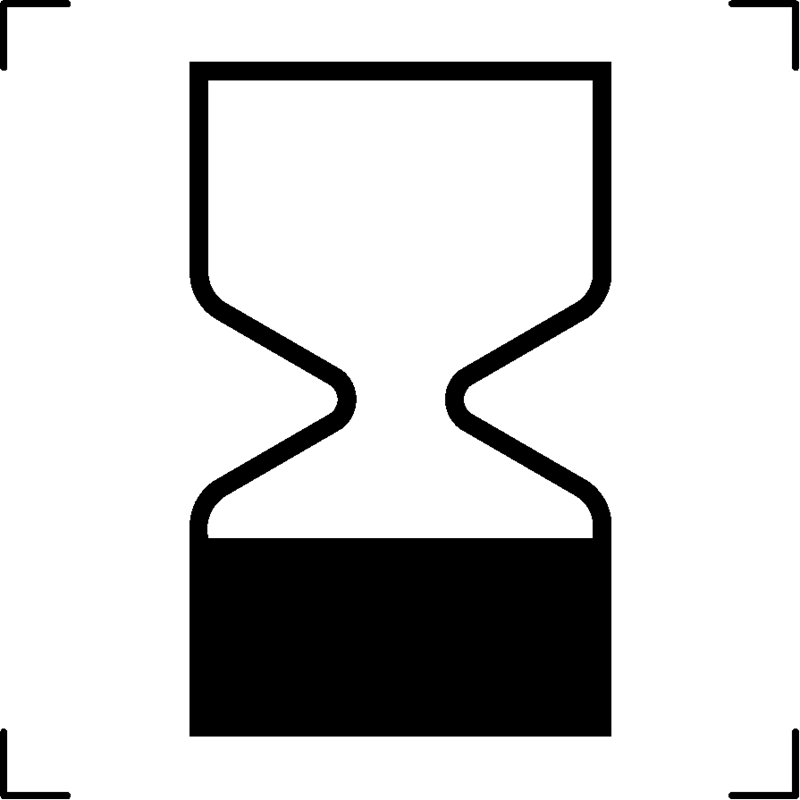 |
Expiry date |
| Medical Device |  |
To signify that the contents of the package are approved under The Medical Devices Regulation 2017/745/EU |
| EU Authorized Representative Role | EU Authorized Representative employed | |
| Swiss authorized representative | The symbol description is as follows: «Indicates the authorized representative in Switzerland» | |
| Batch code | 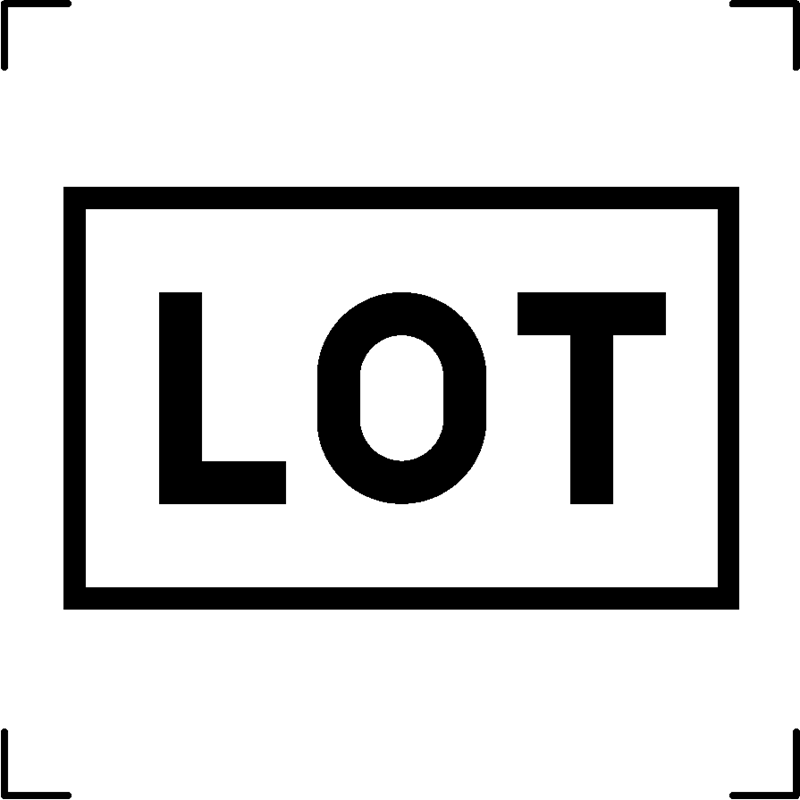 |
Symbol preceding the unique alphanumeric code assigned to a specific set of products, enabling their identification and traceability based on shared production characteristics |
| Catalogue number |  |
Symbol preceding a unique identifier assigned to any transaction |
| Serial number |  |
Symbol preceding the identification number showing the position of a printed or manufactured item in a series |
| Unique device identifier |  |
Identifier bar code or data carrier containing the required UDI information |
| Quantity |  |
Symbol which together with a number shows amount of pieces in a specific packaging |
| Trash box or litter bin or rubbish bin | 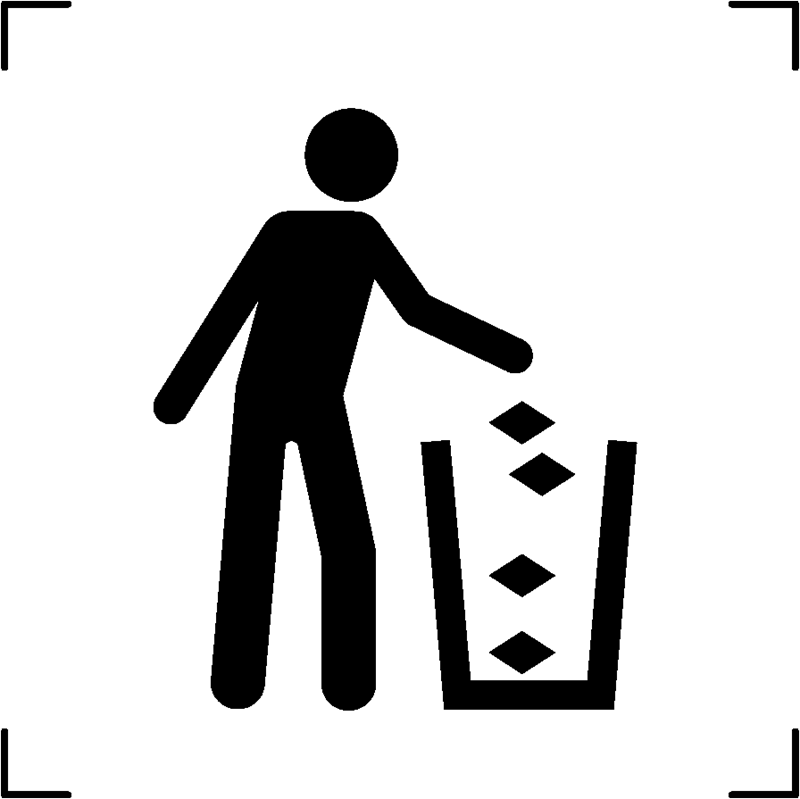 |
To designate a container specifically designed for the collection of waste materials, including trash, litter, or discarded rubbish |
| Keep away from sunlight | 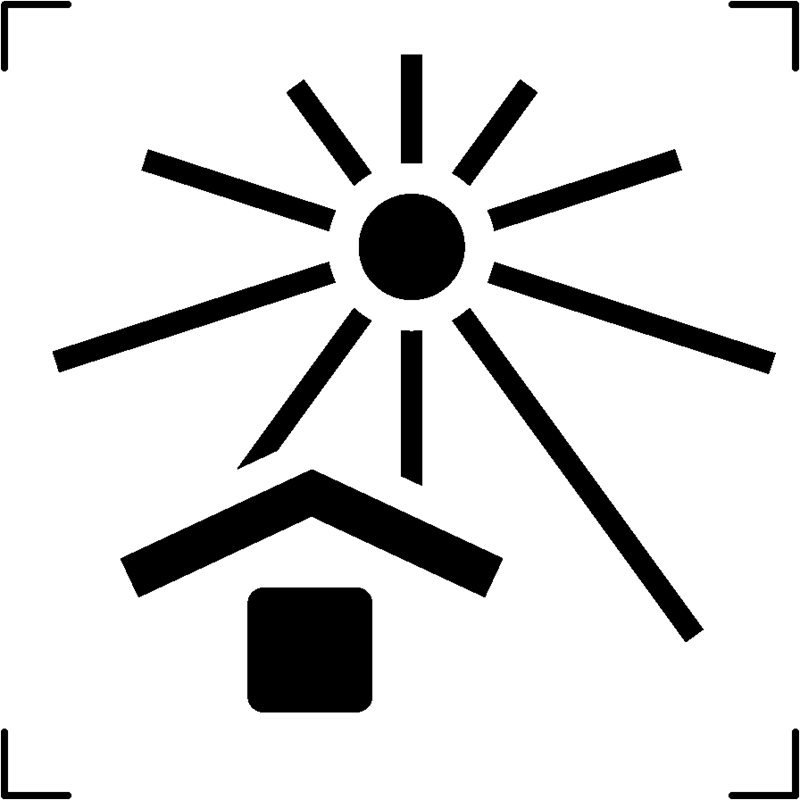 |
To signal that the transportation packaging should be shielded from direct sunlight exposure |
| Keep away from rain |  |
To communicate the requirement for the transportation package to be safeguarded from rain and stored in dry conditions |
| Protect from heat and radioactive sources | 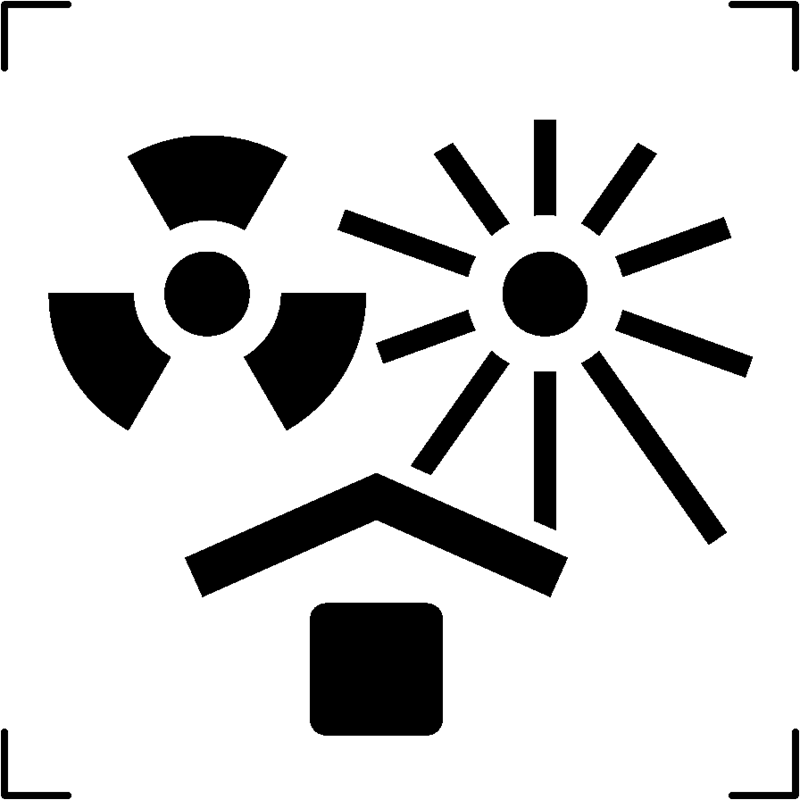 |
To signify that the contents of the package are susceptible to degradation or complete unusability due to exposure to heat or ionizing radiation, emphasizing the necessity of safeguarding them against these factors |
| Do not use if package is damaged | 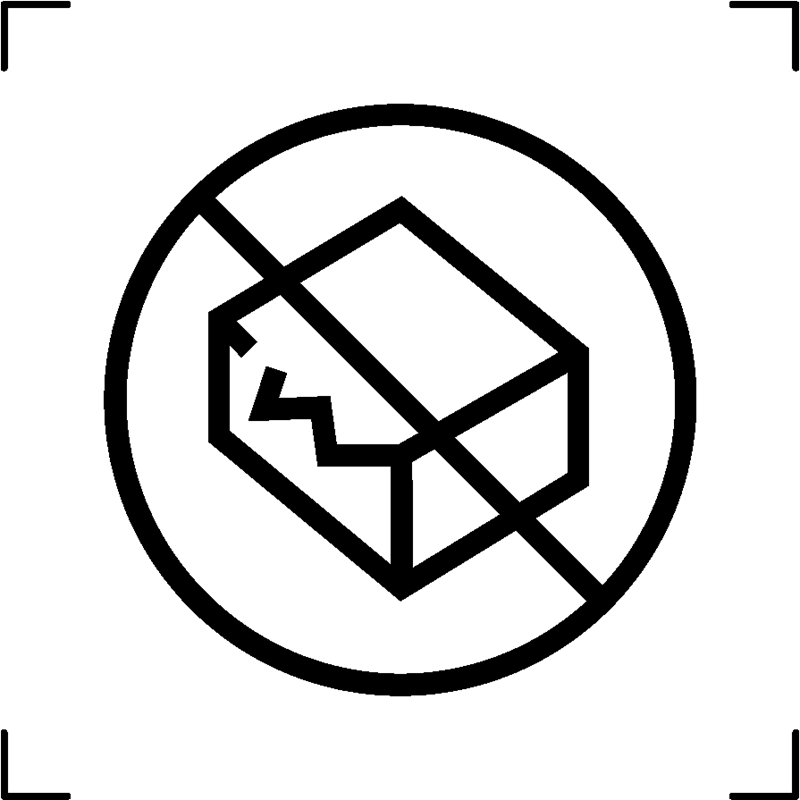 |
To signify that the device should not be utilized in the event of any damage to the packaging containing the device, such as in the case of medical device packaging |
| Operator's manual; operating instructions |  |
To designate the designated storage location for the operator's manual or to indicate information pertaining to the operating instructions. It serves as a reminder that the operating instructions should be consulted when operating the device or controlling it in proximity to the symbol's placement |
| Electronic operator's manual; operating instructions | 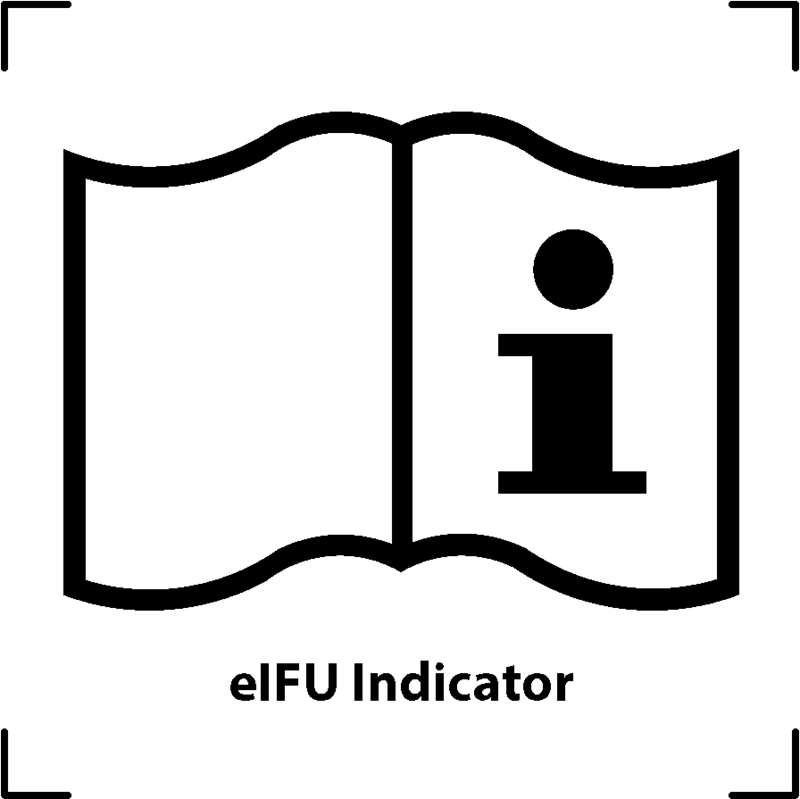 |
To designate the designated electronic storage location for the operator's manual or to indicate information pertaining to the operating instructions. It serves as a reminder that the operating instructions should be consulted when operating the device or controlling it in proximity to the symbol's placement |
| Patient information website | 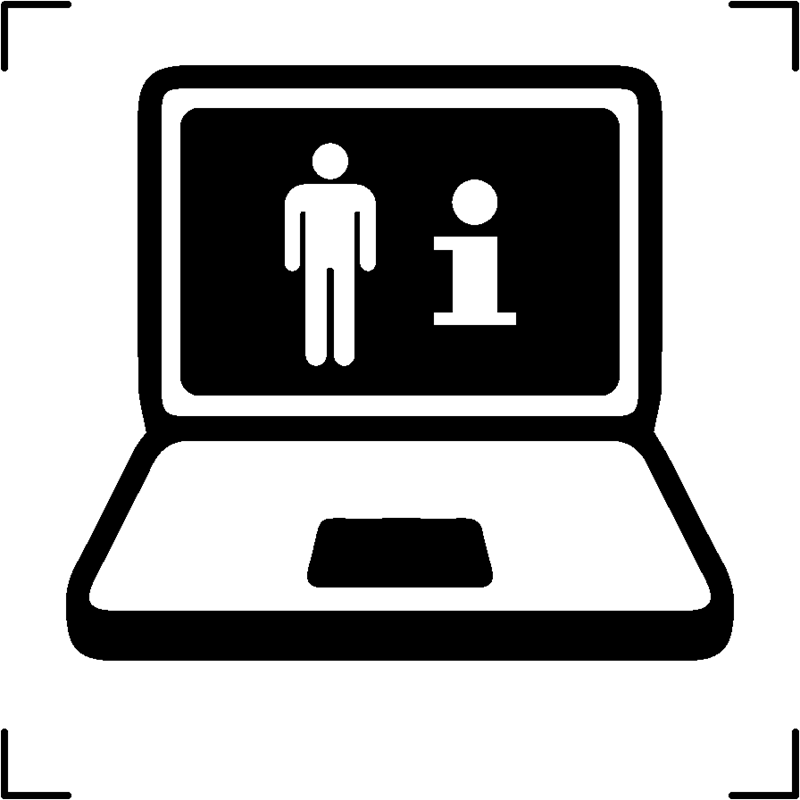 |
To provide a reference to a website where patients can access supplementary information regarding the medical product |
| Single patient multiple use |  |
To signify that the medical device is intended for multiple use on a single patient, allowing for its application in multiple procedures |
| Do not re-use |  |
To signify that the item is intended for single use only and must not be reused or utilized more than once, as seen on packages of medical disposables |
| Caution |  |
To highlight the importance of exercising caution while operating the device or control in close proximity to the symbol's placement. It serves as a reminder that the current situation demands operator awareness or action to prevent undesirable consequences. On the health app quality label: to indicate that the health app necessitates approval from a healthcare professional prior to use |
| Contains or presence of |  |
On medical devices: to signify the presence of the identified product or substance within the equipment |
| Translation |  |
To indicate that the original medical device information has been translated, providing supplementary or replacement information to the original content |
| Repackaging |  |
To identify that a modification to the original medical device packaging configuration has occurred |
| Wash by hand |  |
To signify that the textile article is exclusively suitable for hand washing and should not be cleaned by any other method |
| Wash at 40 degrees |  |
To indicate the the garment is to be washed at 40 degrees Celsius |
| Wash at 60 degrees |  |
To indicate the the garment is to be washed at 60 degrees Celsius |
| Do not bleach | 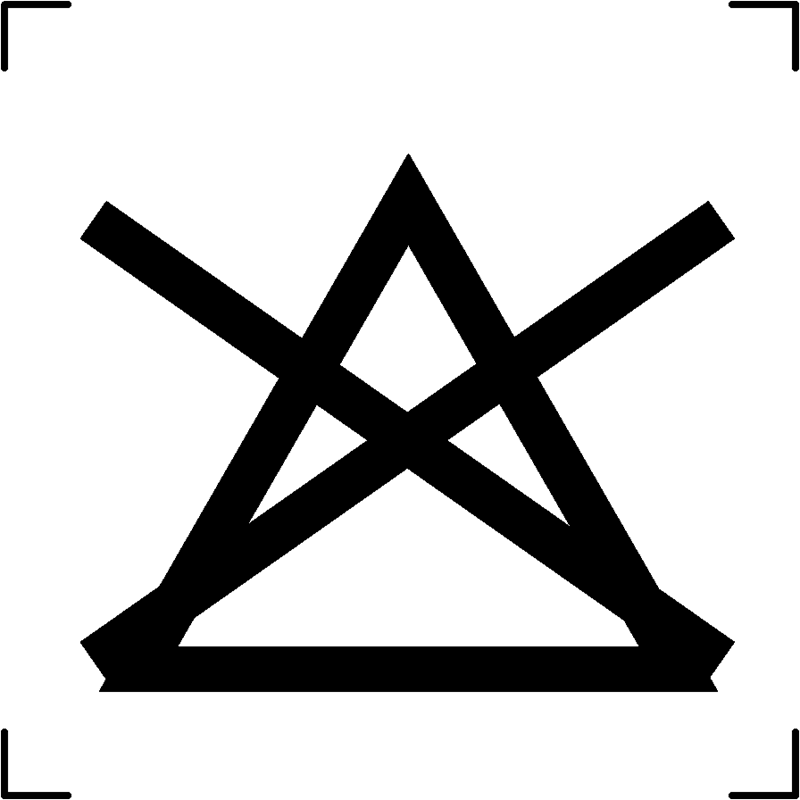 |
To signify that the textile article should not be subjected to any form of bleaching |
| Tumble drying, maximum 60 Celsius |  |
To signify that the textile article is suitable for tumble drying but only at low temperature settings, with a maximum exhaust temperature of 60 degrees Celsius |
| Do not dry clean | 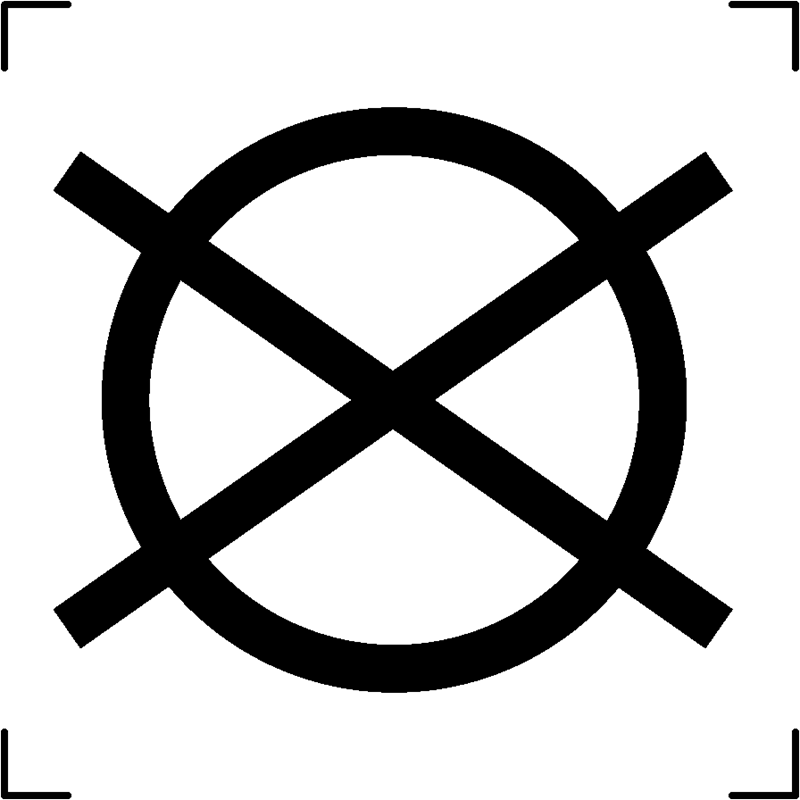 |
To signify that the textile article should not be subjected to dry cleaning |
| Latex free | 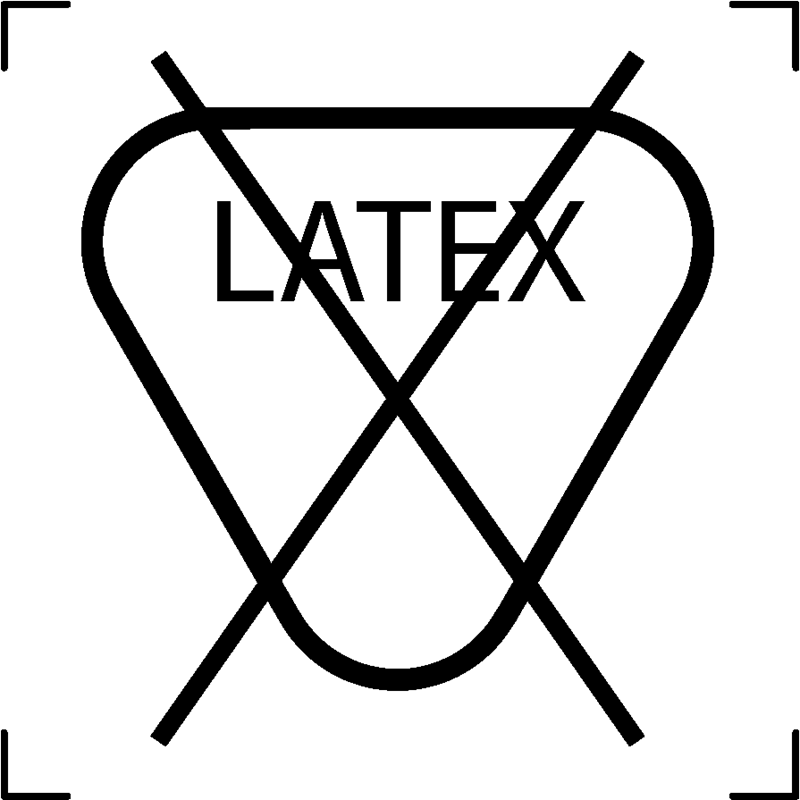 |
To signify that the textile article is free from Latex |
FOLLOW US
At Tytex we cherish input from our users. We want to hear and learn from your story - so please connect and share



